What is Radon?
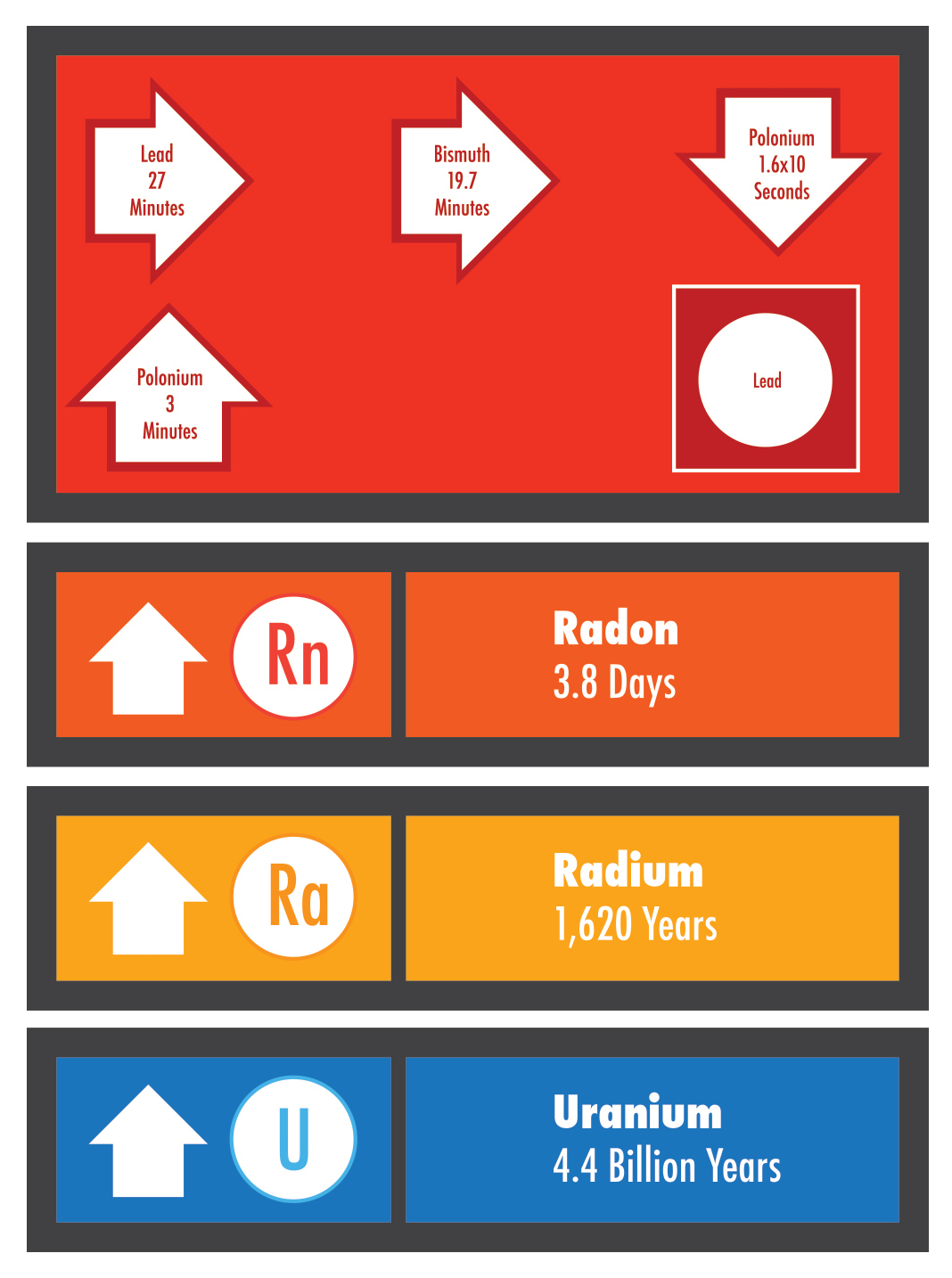
Radon is a gas produced by the radioactive decay of the element radium. Radioactive decay is a natural, spontaneous process in which an atom of one element decays or breaks down to form another element by losing atomic particles (protons, neutrons, or electrons). When solid radium decays to form radon gas, it loses two protons and two neutrons. These two protons and two neutrons are called an alpha particle, which is a type of radiation. Elements that produce radiation are referred to as radioactive. Radon itself is radioactive because it also decays, losing an alpha particle and forming the element polonium.
Elements that are naturally radioactive include uranium, thorium, carbon and potassium, as well as radon and radium. Uranium is the first element in a long chain of decay that produces radium and radon. Uranium is referred to as the “parent” element, and radium and radon are called “daughters” or “progeny.” Radium and radon also form daughter elements as they decay. The progeny of radon are called radon decay products, or RDPs.
The decay of each radioactive element occurs at a very specific rate. How fast an element decays is measured in terms of the element’s “half-life,” or the amount of time for one-half of a given amount of the element to decay. Uranium has a half-life of 4.4 billion years, so a 4.4-billion-year-old rock has only half of the uranium with which it started. The half-life of radon is only 3.8 days.
If a jar were filled with radon, only half of the radon would be left after 3.8 days. But the newly made daughter products of radon (or RDPs) would also be in the jar, including polonium, bismuth, and lead. Polonium is also radioactive. It is this element which is produced by radon in the air and in people’s lungs that can hurt lung tissue and cause lung cancer.
Radon Measurements
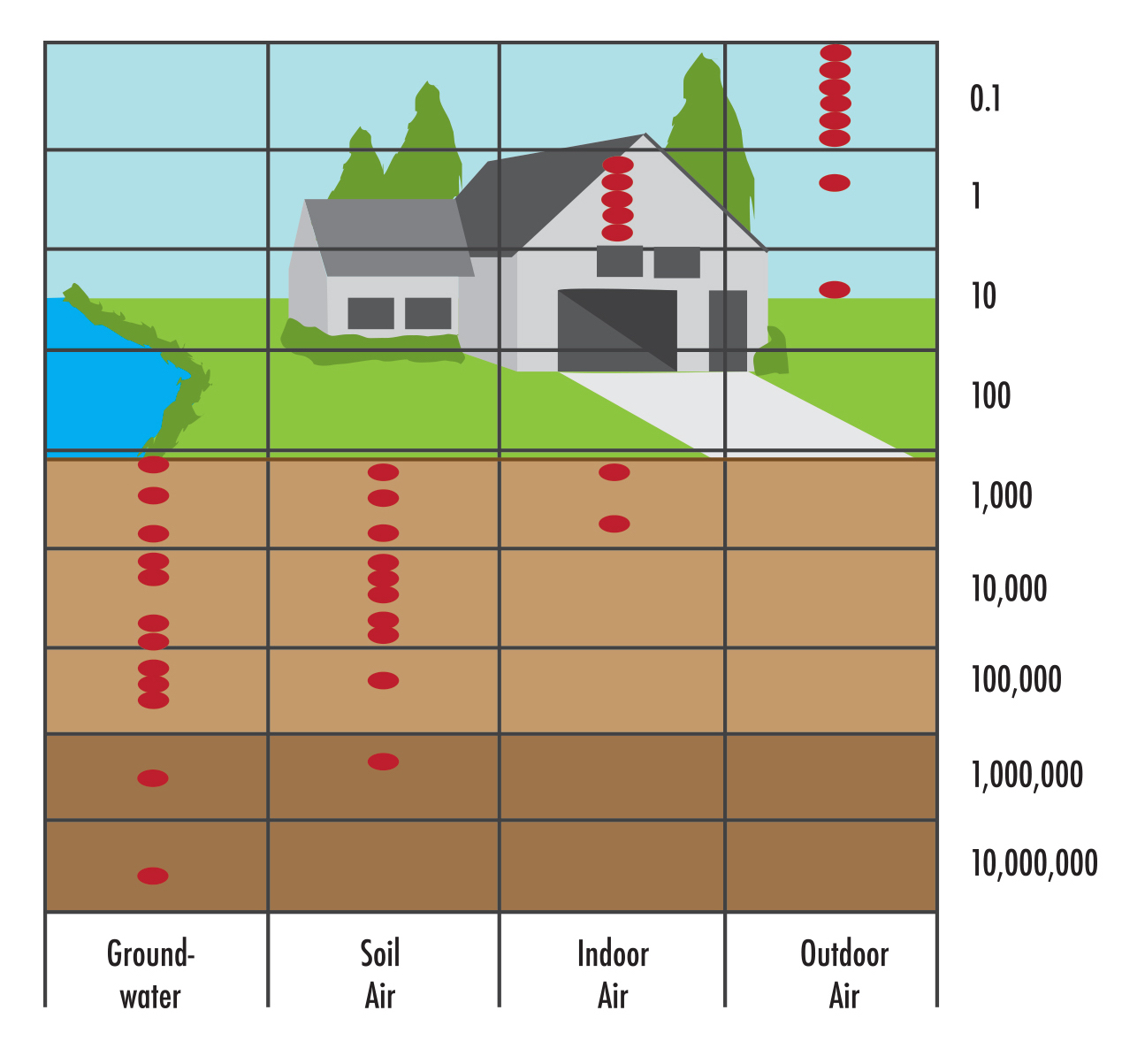
Radioactivity is commonly measured in picocuries (pCi). Because the level of radioactivity is directly related to the number and type of radioactive atoms present, radon and all other radioactive atoms are measured in picocuries. For instance, a house having 4 picocuries of radon per liter of air (4 pCi/L) has about eight or nine atoms of radon decaying every minute in every liter of air inside the house. A 1,000-square-foot house with 4 pCi/L of radon has nearly 2 million radon atoms decaying inside it every minute. Radon levels in outdoor air, indoor air, soil air, and groundwater can be very different. Outdoor air ranges from less than 0.1 pCi/L to about 30 pCi/L, but it probably averages about 0.2 pCi/L. Radon in indoor air ranges from less than 1 pCi/L to about 3,000 pCi/L, but it probably averages between 1 and 2 pCi/L. Radon in soil air (the air that occupies the pores in soil) ranges from 20 or 30 pCi/L to more than 100,000 pCi/L; most soils in the United States contain between 200 and 2,000 pCi of radon per liter of soil air. The amount of radon dissolved in groundwater ranges from about 100 to nearly 3 million pCi/L.
Conclusion
Radon is a naturally-occurring radioactive gas. Radon itself is radioactive because it also decays, losing an alpha particle and forming the element polonium. Radioactivity is commonly measured in picocuries (pCi). Groundwater, soil air, indoor air, and outdoor have different acceptable levels of radon gas. An IAC2 Certified Consultant will help you determine the levels of radon in your home or commercial building.